(3519) 580-155, mail@ntpf-etalon.ru
|
|
Ferrochromium Nitride SH-SynthesisM. Kh. Ziatdinov1, I. M. Shatokhin2
1 - Tomsk State University
2 - NTPF Etalon Co., Ltd (Magnitogorsk, Russia)
Ferrochromium nitride is a unique alloying material suitable for use in the production of corrosion-resistant nitrogen steel. Ferrochromium nitride has been produced since the 1930s, when it was employed primarily to introduce considerable quantities of nitrogen in chrome steel. In the first research on alloying and nitriding chromium alloys, the ingot structure of chrome steel (24–28 % Cr, ~0.25 % N) was improved by transcrystallization. With the growing global militarization and the division into two armed camps that begin in the 1930s, raw materials for the production of high-quality steel - including stainless steel - were in very short supply. Nickel was a particular concern. The search for alternatives kick-started research on nitrogen-bearing stainless steel. Alloying with nitrogen considerably reduced nickel consumption and, in some cases, entirely eliminated the need for nickel. In such alloys, nitrogen greatly stabilizes the austenite. In terms of its austenite-forming properties, 0.1 % N is equivalent to ~2.0 % Ni. In contrast to carbon, nitrogen also greatly expands the gamma region but also reduces the corrosion resistance of steel. Moreover, it is found that introducing nitrogen considerably improves the strength of steel, without loss of plasticity. Nitrogen is a gas of practically unlimited availability. It is readily accessible, without regard to national boundaries, and is environmentally safe. Collecting nitrogen is inexpensive and simple. Steel may be alloyed either directly with natural gaseous oxygen or by artificial nitrogen carriers, such as compounds or alloys. In terms of modern-day steel production, with a reliance on ladle treatment, the first option might seem preferable at first glance. Indeed, in many cases, treatment of the steel melt with gaseous nitrogen gives a positive result. However, we should note the deficiencies of this technology: inability to attain the maximum nitrogen concentration; instability of nitrogen assimilation, which depends on many factors; and, in some cases, excessive duration of nitrogen injection, which is accompanied by supercooling of the metal. In practice, therefore, introduction of nitrogen by means of solid additives is also employed. Recently, the most common approach is to use powder wire filled with various nitrogen compounds. Since the wire is added to the metal in the final stages of smelting, its consumption will be a minimum, whereas the content of nitrogen in the filler will be a maximum. At present, ferrochromium nitride is used for the production of many corrosion-resistant steels. The commonest approach is the production of austenitic steel on the basis of Cr–Mn and Cr–Mn–Ni alloys. Ranges of such steel are standardized in most industrial conditions. Series-200 steel (such as UNS20100, UNS20200, and UNS20500) is widely used. Such steel first appeared in the 1950s and quickly dominated the US market. China and India - the countries that are most rapidly developing - are the largest producers of nitrogen-bearing corrosion-resistant steel. The output of such steel is constantly rising and currently accounts for 10 % of total global steel production. In Asia, by contrast, it accounts for ~20 % of production. Chromo-manganese nitrogen steel is used in the construction, transportation, chemical, and food industries and also in the auto industry, in home appliances, and in ship-building. Besides series-200 steel, which is mass-produced and widely used, specialized high-nitrogen steels have been developed. Because of their distinctive properties, these steels have no real counterparts. For example, 08-12X18AГ18 steel with ~0.5 % N is used in the manufacture of bandage rings for turbogenerators; 55X21Г9AH4 steel (with 0.3–0.6 % N) is used in auto-engine valves; and other steel operates in cryogenic conditions and is resistant to sea water. In fact, only alloying with nitrogen simultaneously increases the strength, ductility, and corrosion resistance of the metal. Nitrogen reacts with chromium and its alloys only at high temperature. Therefore, current nitriding methods are based on furnace technology. There have been attempts to produce nitrogen-bearing chromium alloy by ladle reduction. However, this technology is not widely used, since it is very expensive, and the product is characterized by a low nitrogen concentration - hardly more than 1 %. In furnace conditions, nitriding may occur at different temperatures, which will affect the product quality. Below the melting point of ferrochrome, solid-phase nitriding occurs, with the formation of a sintered product or powder. In nitriding a melt, a liquid-phase process yields a cast product. In the first case, the maximum possible nitrogen content of the product is determined by its content in chromium nitride; in the second, by contrast, it is determined by the solubility of nitrogen in chrome melt. The general requirements imposed today, on the basis of 70 years of experience, are as follows. The nitrogen content specified for the grade of steel must be stably maintained with the minimum consumption of alloying additives, and without the pronounced introduction of impurities. In practice, this means that alloys with higher nitrogen content and lower impurity content is used so as to obtain maximum nitrogen assimilation. It is important to employ an economical method of alloy production. Whereas the nitrogen concentration in the alloying additives is determined by the production technology employed, the degree of nitrogen assimilation depends on numerous factors: the method of introducing the alloying additives in the melt, the presence of impurities, the grade composition of the steel, etc. Since the onset of mass reduction of ferrochromium nitride in the 1950s, the nitrogen concentration has steadily increased, and the content of carbon and other impurities has declined. This is reflected both in production standards and in the specific composition of the alloying additives employed. For example, whereas State Standard GOST 4757–49 specified only ferrochromium Khrn1 with ~1 % N, the current standard includes eight grades with a minimum nitrogen content between 1 and 8 %. In the world today, ferrochromium nitride is mainly produced by vacuum heat treatment; the result is a sintered product with a high nitrogen content. Sometimes, this material is remelted to obtain a dense ingot, but with a low nitrogen content. The raw material for nitriding is either low-carbon ferrochrome or carbon ferrochrome. In the latter case, the alloy is decarburized before saturation with nitrogen. In the United States, a classic simplex process is used for this purpose. In the initial stage, the product contains 2–5 % N. After remelting, a molten product with 0.75–2.0 % N is obtained. The nitrogen concentration in ferrochromium nitride produced today by the simplex process is ~7.5 % (according to Eramet Comilog data). Table 1 presents information on ferrochromium nitride produced in different countries. Table 1. Approximate chemical composition of ferrochromium nitride, %*
*The minimum content is given for nitrogen and chromium, while the maximum values are given for the other elements
In the Soviet Union, an improved production method for low-carbon ferrochrome in vacuum-heating furnaces was developed. It may also be used to produce sintered high-nitrogen ferrochrome. The technology was introduced at Aktyubinsk ferroalloy plant. In the 1980s, the output of ferrochromium nitride reached 1500–2000 t/yr. The product contained up to 8 % N and was used to smelt a wide range of nitrogen-bearing chrome steel. The vacuum-heating technology was also characterized by high productivity and low energy consumption. (The complete cycle was ~9 days; power consumption was 9500 kW-h/t.) On account of the high sensitivity of the process to temperature and pressure differences, the nitrogen was nonuniformly distributed over the volume, for the whole stack and also for individual briquets. Efforts to obtain low carbon content (0.03–0.06 %) led to excessive residual oxygen content in the product, which impaired nitrogen assimilation and increased the content of nonmetallic nclusions in the steel. Chromium nitride, which is used primarily for the smelting of nitrogen-bearing nickel and cobalt superalloys, is obtained by high-temperature treatment of chromium powder in the solid state. To this end, either electrolytic metal (Eramet Comilog, United States) or metal produced by a thermal technology (Delachaux, France; LSM, Britain) is employed. The composition of the product from different manufacturers is similar and corresponds, in terms of nitrogen content, to the more thermostable nitride Cr2N (Table 2). Table 2. Approximate chemical composition of chromium nitride, %
*The minimum content is given for nitrogen and chromium, while the maximum values are given for the other elements
Self-propagating high-temperature synthesis is a practical alternative to vacuum heating in the production of chromium nitride and ferrochromium nitride. This technology has already been successfully used in the production of ferrosilicon nitride. Benefits of self-propagating high-temperature synthesis include low energy consumption, maximum production rate, and, in particular, the ability to obtain materials with properties that are unattainable by other methods. Self-propagating high-temperature synthesis depends on a strongly exothermal reaction of the batch components. For the Cr–N system, this condition is satisfied. Considerable heat is liberated in the formation of the nitrides Cr2N and CrN: 105.3 and 117.9 kJ/mole, respectively. The corresponding adiabatic combustion temperatures are 1290 °C for Cr2N and 2060 °C for CrN, according to the method in [13]. Although the formation of chromium nitrides is considerably less exothermal than the corresponding reactions for metals of groups IV and V (for example, for Ti and Zr, Tad > 4500 °C; for VN and NbN, Tad ≈ 3200 °C), it is perfectly satisfactory for self-propagating high-temperature synthesis. Moreover, by selecting the optimal conditions of synthesis, combustion is possible in the even less exothermal ferrochromium–nitrogen system. Since practically no heat is liberated in the formation of iron nitrides and the nitrides Fe4N and Fe2N are thermal unstable, iron serves as thermal ballast. PKh1S chromium powder produced by the calciumhydride method (Technical Specifications TU 14-1-1474–75) and PKhA97.5 chromium powder produced by the alumothermal method (Technical Specifications TU 14-00186482-051–2005) are used in laboratory experiments, as well as PFN low-carbon alumothermal ferrochrome (State Standard GOST 4757–91). The experimental method was described in; it is analogous to the nitriding of other metals and ferroalloys by combustion. The most important parameter of such combustion is the nitrogen pressure in the vicinity of the hot powder. In Fig. 1, the combustion rate of ferrochrome powder with 75.6% Cr is plotted as a function of the nitrogen pressure; for comparison, the analogous curves for standard alloys are shown: ferrotitanium (FTi70, 70.6 % Ti); ferrovanadium (FVa75U0.1; 71.6 % V); and ferroniobium (FN660; 60.6 % Nb). In all cases, powder with a particle size of <0.04 mm is used. The powder is burned without forced compaction in cylindrical gas-permeable casings of various diameters. The porosity of the initial samples is ~60 %. In all cases, the combustion rate increases with increase in the pressure, on account of the better conditions for nitrogen filtration. As would be expected, combustion is slowest for ferrochromium. This is due both to the less exothermal nitride formation for chromium than for metals of groups IV and V and to the fact that the reaction of ferrochrome and nitrogen in the combustion wave is not a solid-phase process. The melting point of low-carbon ferrochrome is 1550–1670 °C. Even if we take into account that the nitrogen dissolved in the ferrochrome somewhat reduces its melting point, this temperature will remain above the combustion temperature. In the given pressure range (1.0–12.0 MPa), the thermocouple readings of the combustion temperature are no higher than 1300 °C. Metallographic analysis of the combustion products confirms the absence of melting in the Cr–Fe–N system. 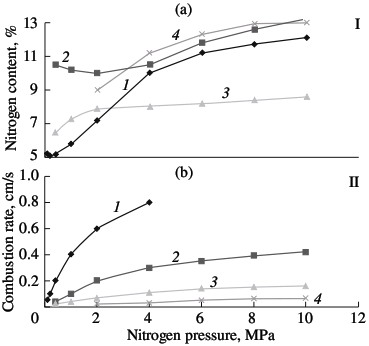 Fig. 1. Influence of the nitrogen pressure on the degree of nitriding (I) and combustion rate (II) of ferroalloys:
(1) ferrotitanium; (2) ferrovanadium; (3) ferroniobium; (4) ferrochrome. Solid-phase processes usually facilitate extensive nitriding of both metals and ferroalloys. The possibility of stage-by-stage saturation of the powder with nitrogen was observed earlier in the combustion of ferrovanadium and ferrosilicon. Most of the nitrogen is absorbed by the alloy in the synthesis wave, sometimes in conditions of laminar combustion. The unreacted portion of the ferroalloy reacts with nitrogen in conditions of bulk combustion. This facilitates the retention of high porosity at the combustion front. As a result, the nitrogen content in the products may be increase by 10–40 %, depending on the conditions of combustion and the composition of the initial alloy. Note, however, that, even in the most optimal combustion conditions, the maximum degree of nitriding is not attained in the nitriding of ferrochrome. For alloys with 75.6 % Cr, the limiting calculated nitrogen concentration when all the chromium is converted to nitride CrN1.0 is around 16.8 % N. The actual maximum nitrogen content in the ferrochrome is ~13.0 % N. Thus, the actual degree of nitriding is hardly more than 77 % of the calculated value. Close to 100 % of the maximum nitriding is obtained in the combustion of metallic chromium powder in nitrogen. The combustion rate and temperature and the degree of nitriding of calcium-hydride chromium powder with a particle size of <0.02 mm are plotted in Fig. 2. In the pressure range 0.1–0.9 MPa, all three parameters increase monotonically. At the maximum pressure, practically all the chromium has been converted to CrN. X-ray diffraction data show that the product obtained at pressures above 3.0 MPa consist of a single phase. At the minimum pressure (0.1–0.2 MPa), the burning material consists mainly of chromium seminitride Cr2N; lines corresponding to CrN and free chromium are also seen. With increase in pressure, the content of CrN rapidly rises. 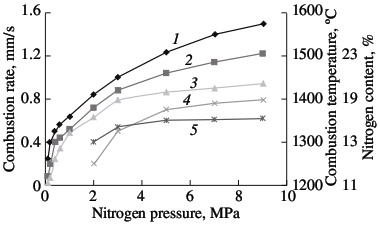 Fig. 2. Influence of the nitrogen pressure on the combustion rate (1, 4), degree of nitriding (3, 5) and combustion temperature (2):
(1, 2, 3) chromium; (4, 5) ferrochrome Alumothermal chromium powder is less active than calcium-hydride powder. The high reactivity is mainly a result of the large surface area of the powder, due to its small size. Microscopic analysis shows that, in calcium-hydride chromium, even relatively large particles (0.04–0.05 mm) in fact consist of aggregations of smaller particles (no greater than ~0.01 mm). At the same time, particles larger than 0.01 mm predominate in alumothermal chromium powder that passes through a 0.04-mm screen. An analogous pattern is observed for ferrochromium powder. On account of the high strength of the low-carbon ferrochrome and metallic chromium produced by the alumothermal method, considerable energy consumption is required in order to obtain fine powder. It is evident from Fig. 2 that the combustion rate and the degree of nitriding of alumothermal chromium powder are considerably less than those of calcium-hydride powder. Since the conversion depth of the alumothermal chromium is between 70.7 % (15.0 % N) and 82.1 % (17.4 % N), the combustion product consists of two phases, including two chromium nitrides. In contrast to chromium, the combustion products of ferrochrome always consist of multiple phases. X-ray diffraction data indicate α iron, a double nitride (Fe, Cr)2N, and the mononitride CrN. Over the whole pressure range, this phase composition remains basically the same. With increase in nitriding, thanks to higher pressure, the content of the mononitride and free iron increases. The same pattern was observed earlier in furnace synthesis. On the basis of the results of laboratory experiments and tests at NTPF Etalon Co., Ltd production facility (Magnitogorsk), an industrial system for the nitriding of chromium-based alloying additives by self-propagating high-temperature synthesis has been developed and introduced. Four fundamentally new high-nitrogen alloying materials are proposed here: sintered chromium nitride, fused chromium nitride, sintered ferrochromium nitride, and fused ferrochromium nitride. Table 3 presents the chemical and phase composition of these materials, as well as their density. The macrostructure of the new materials is shown in Fig. 3. Table 3. Chemical composition and properties of alloys produced by self-propagating high-temperature synthesis*
*The minimum content is given for nitrogen and chromium, while the maximum values are given for the other elements
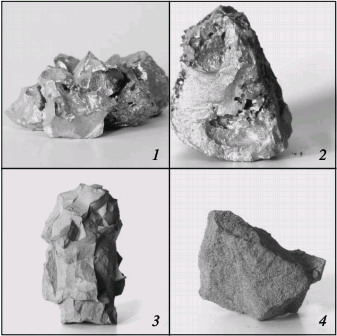 Fig. 3. Macrostructure of new alloying materials:
(1, 2) fused and sintered chromium nitride; (3, 4) fused and sintered ferrochromium nitride Ferrochromium nitride is used in smelting nitrogen-bearing corrosion-resistant steel and other chromium-bearing alloys with nitrogen. Chromium nitride is recommended for predominant use in smelting high-nitrogen chromonickel superalloys and also for high-alloy corrosion-resistant steel with the maximum nitrogen concentration. Fused ferrochromium nitride and chromium nitride may be effectively used in chunk form for direct alloying in the furnace or in the ladle at melt introduction. The sintered materials may be introduced not only in the traditional form but also as powder wire. On account of the high nitrogen concentration, the consumption of such wire will be minimal with high and stable nitrogen assimilation by the melt. The new nitrogen-bearing chromium materials have been successfully tested at numerous Russian and non-Russian plants in steel production. The required nitrogen content in the steel varies widely: from 0.04 % to 0.6 %. In particular, 12X18AГ18, 35X2AФ, 55X21Г9AH4, 110Г13XФAЛ, and related steel has been produced, as well as chromonickel alloy with ~0.5 % N. The metal is smelted in electrofurnaces, using various chromium and ferrochromium nitrides, containing 9.0–19.5 % N. In all cases, the degree of nitrogen assimilation is more than 90 %. Thus, industrial technology for the production of nitrogen-bearing chromium-based alloying materials on the basis of self-propagating high-temperature synthesis has been developed for the first time in Russia. The chromium-based alloying materials obtained at the Etalon production facility on the basis of self-propagating high-temperature synthesis have no counterparts anywhere in the world. Chromium and ferrochromium nitrides, which combine a high nitrogen concentration and maximum density, permit the economical smelting of a wide range of nitrogen steel. Published in "Steel In Translation" Volume 39, Number 9, September 2009 (article in pdf)
Ferrochromium and chromium nitride, produced by NTPF Etalon Co., Ltd: Other publications about SHS |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
