(3519) 580-155, mail@ntpf-etalon.ru
|
|
Ferrovanadium Nitride SH-Synthesis. Part 2Previous article's part - Ferrovanadium Nitride SH-Synthesis. Part 1 Two methods may be employed to observe the stages of nitrogen absorption in filtrational alloy combustion. The first is based on combustion in special equipment for self-propagating high-temperature synthesis, where the change in sample mass during the process is continuously recorded. The corresponding curves for industrial ferrovanadium are shown in Fig. 3. It is evident that the variation in sample mass over time for FeV80 and FeV60 alloys is qualitatively different from that for FeV50 and FeV40 alloys. The gravimetric curves for the σ-alloys have a point of discontinuity beyond which the sample mass remains constant. The gravimetric curves for the α-alloys (FeV80 and FeV60) have no such discontinuity. Beyond the linear section corresponding to layer-by-layer combustion, the curves smoothly tend to a maximum (complete bulk combustion). Thus, we may say that, in the nitriding of ferrovanadium alloy corresponding to the range of existence of α-phase (>55% V), two-stage absorption of nitrogen occurs. In the first stage, ~75% N is absorbed in conditions of layer-by-layer combustion; the remaining nitrogen is absorbed in α-ferrovanadium during final bulk combustion. An alloy whose vanadium content corresponds to the range of existence of σ-phase (35–55% V) will be nitrided in a single-stage process. All the nitrogen within the intermetallide alloy (vanadium mononitride) is absorbed during layer-by-layer combustion. 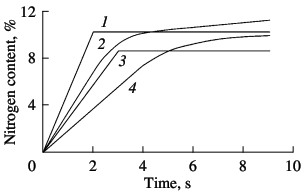 Fig. 3. Parametric nitriding curve of ferrovanadium:
(1) FeV50; (2) FeV80; (3) FeV40; (4) FeV60. The different mechanisms of nitrogen saturation for α- and σ-ferrovanadium are confirmed by layer-by-layer chemical analysis of quenched samples. By sharp cooling of burning samples, combustion is halted. The samples are then subjected to chemical analysis by the Kjeldahl method. The samples are taken from layers directly adjacent to the combustion front on the nitriding-product side (at a distance of ~0.5 mm). The results are shown in Fig. 4: 1) calculated values of the maximum possible nitrogen content with complete conversion of vanadium to stoichiometric mononitride; 2, 3) nitrogen content in the combustion products of the alloys and mixtures, respectively, with slow cooling of samples; 4) nitrogen concentration in quenched samples. Comparison of curves 2 and 4 indicates that, whereas the nitrogen concentration in quenched samples is markedly less than that in slowly cooled samples for vanadium and α-ferrovanadium (alloys with 78.8 and 59.2% V), the corresponding nitrogen concentrations are practically the same for σ-ferrovanadium (alloys with 52.4 and 41.6 % V). This confirms the conclusion derived from experiments with continuous weighing of samples during nitriding. In the combustion of alloys corresponding to the σ-intermetallides, nitrogen is absorbed in a single stage during layer-by-layer combustion. If ferrovanadium with the structure of an α-solid solution is nitrided, two-stage absorption is observed. Nevertheless, the conversion of vanadium to nitride is greater in σ-ferrovanadium than in α-ferrovanadium: 83 and 84 % for FeV50 and FeV40 alloys; and 68 and 79 % for FeV80 and FeV60 alloys. Formetallic vanadium, the corresponding figure is 58 %.  Fig. 4. Influence of vanadium content in ferrovanadium on
the degree of nitriding. As for other ferroalloys and metals, the nitriding of ferrovanadium at pressures up to 15 MPa occurs as filtrational combustion. The distinguishing feature of such self-propagating high-temperature synthesis is a strong dependence of the combustion characteristics and the composition of the reaction products on the nitrogen pressure, the fineness of the initial powder, and the permeability of the powder batch. The corresponding curves of FeV80, FeV60, FeV50, and FeV40 alloys are shown in Figs. 5–7. In all cases, increasing the nitrogen pressure, reducing the particle size of the ini-tial alloys, and reducing the porosity of the nitrided samples boosts the combustion rate. The quantity of nitrogen in the reaction products is higher on nitriding coarser powder, when using high nitrogen pressure, and when using more permeable samples. These results are expected and qualitatively agree with the data obtained in the combustion of model alloys similar in composition to the industrial alloys. Analogous results were obtained earlier for the nitriding of metal and ferroalloy powders. 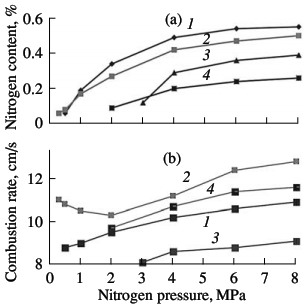 Fig. 5. Influence of the nitrogen pressure on the combustion rate (a) and degree of nitriding (b) of ferrovanadium: (1–4) as in Fig. 3
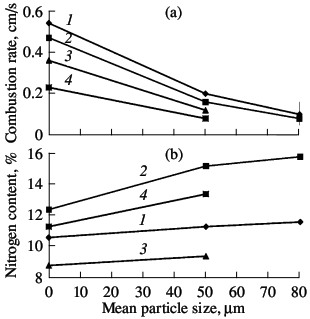 Fig. 6. Influence of the size of the powder particles on the
combustion rate (a) and degree of nitriding (b) of ferrovanadium: (1–4) as in Fig. 3 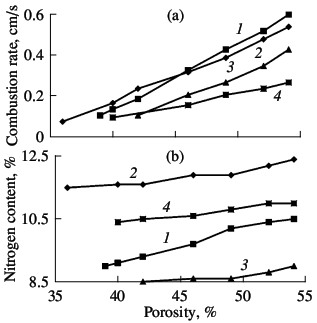 Fig. 7. Influence of the sample porosity on the combustion rate (a) and degree of nitriding (b) of ferrovanadium: (1–4) as in Fig. 3
The combustion temperature in nitriding ferrovanadium is measured by means of VR5/VR20 tungsten–rhenium thermocouples. Typical temperature profiles for α- and σ-ferrovanadium are shown in Fig. 8. The temperature in the combustion wave of the intermetallides alloy grows very rapidly and then remains constant for a long time. The combustion temperature of α-ferrovanadium is markedly less, but the final temperature is higher. The maximum combustion temperature varies quite widely, depending on the nitriding conditions: 1780–2060 °C for FeV80; 1630–1830 °C for FeV60; 1480–1560 °C for FeV50; and 1420–1490 °C for FeV40. Analysis of the results shows that, in general, the maximum temperature correlates well with the nitrogen content in the product: if more nitrogen is absorbed by the alloy, there will be more heating on combustion. The qualitatively different temperature profiles indicate different combustion mechanisms of the α- and σ-ferrovanadium: single-stage nitrogen absorption by the intermetallide alloy and two-stage absorption by the solid-solution alloy. 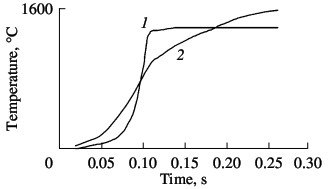 Fig. 8. Temperature profiles of ferrovanadium combustion:
(1) σ-FeV; (2) α-FeV. We know that the economic efficiency of vanadium increases when certain vanadium alloys are used, including nitrogen-bearing alloys. In the present work, we investigate the possibility of obtaining complex nitrided alloys that contain vanadium as well as manganese, niobium, and silicon. These components are obtained from powders of standard industrial alloys: ferroniobium, ferromanganese, and ferrosilicon. Although the heat of formation of nitrides of silicon (Si3N4), niobium (NbN), and manganese (Mn3N2) are completely comparable with those of vanadium nitrides, their reaction with nitrogen is much less intense. As we see in Fig. 9, all three additives reduce the combustion rate of ferrovanadium. Manganese and niobium somewhat reduce the nitrogen concentration in the reaction products; ferrosilicon, by contrast, considerably increases the nitrogen concentration. Thus, self-propagating high-temperature synthesis permits the production of any complex alloying composite containing V, Nb, Mn, and/or Si on the basis of the corresponding standard-ferroalloy powder. The nitrogenconcentration is close to the maximum for the chosen composition. 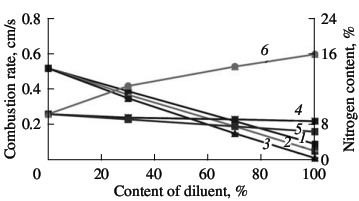 Fig. 9. Influence of the ferroalloy dilution on the combustion rate (1–3) and degree of nitriding (4–6) of ferrovanadium: (1, 4) FeNb; (2, 5) Mn; (3, 6) FeSi.
Laboratory data for industrial ferrovanadium alloys permit the development of a production technology for ferrovanadium nitride on large equipment (working volume 0.15 m3). The combustion rate in such industrial reactors for self-propagating high-temperature synthesis is determined by means of a flow-rate meter mounted at the inlet. After combustion is initiated by sending an electric pulse to the ignition unit, the nitrogen flow rate sharply rises, and quickly reaches a constant value. Nitriding at constant flow rate corresponds to steady layer-by-layer combustion. The flow-rate meter records the end of this stage from the sharp change in the rate of nitrogen absorption. In nitriding σ-ferrovanadium, the nitrogen flow rate falls practically to zero, as confirmed by the absence of final bulk reaction. In the nitriding of α-ferrovanadium, after rapid decline in the nitrogen absorption rate recorded by the flow-rate meter, saturation of the alloy with nitrogen continues at a lower and constantly decreasing rate. The combustion rates of ferrovanadium measured in this way in the industrial reactor are close to those obtained in the laboratory. Thus, the combustion of FeV50 alloy at 12 MPa lasts 5–8 min, which corresponds to a rate of layer-by-layer combustion of 0.2–0.3 cm/s. The absorption rate of nitrogen by ferrovanadium is ~0.1 m3/s. The nitrogen concentration in the combustion products of industrial ferrovanadium depends on the initial alloy, the nitrogen pressure in the reactor during combustion, the fineness of the powder, and various other factors and may vary within the range 8–16 %. The traditional method is employed to attain the maximum possible (limiting) nitrogen content in the combustion products. To reduce the exothermal character of the batch, reaction products are usually added. This reduces the temperature of the process, on the one hand, and, on the other, prevents particle coagulation at the combustion front and reduces the permeability behind the front, thereby creating conditions for maximum conversion of the metal to nitride in the final nitriding stage. The influence of dilution with reaction products is investigated for the combustion of FeV80 and FeV60 ferrovanadium. The nitrogen content in the combustion product is plotted in Fig. 10 as a function of the quantity of nitriding products in the initial mixture. The particle size is the same in all the powders (0.04 mm). FeVN80 ferrovanadium nitride powder synthesized from FeV80 alloy contains 12.1 % N; FeVN60 ferrovanadium nitride from FeV60 alloy contains 10.9 % N. It is evident from Fig. 10 that the limiting nitrogen concentration in FeV80 corresponds to the addition of 30 % FeVN80 to the batch, while the limiting nitrogen concentration in FeV60 corresponds to the addition of 15 % FeVN60 to the batch. The combustion rate of mixtures diluted with the final product declines rapidly with increase in diluent concentration. 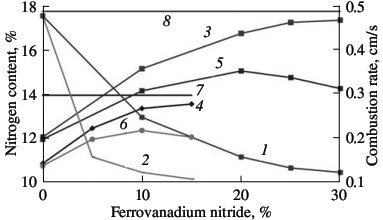 Fig. 10. Influence of the ferroalloy dilution on the combustion rate (1, 2) and degree of nitriding (3–6) of ferrovanadium: (1, 3, 5) FeV80 (FeVN80); (2, 4, 6) FeV60 (FeVN60); (3, 4) total nitrogen content; (5, 6) increment in nitrogen on combustion; (7, 8) limiting nitrogen content for FeV60 (7) and FeV80 (8).
Two types of ferrovanadium nitride produced by self-propagating high-temperature synthesis (SHS) have been developed for industrial use: fused and sintered ferrovanadium nitride. Fused SHS ferrovanadium nitride is used for traditional chunk alloying of steel in the ladle or directly in the furnace. Sintered SHS ferrovanadium nitride serves as an effective filler for powder wire in adjusting the nitrogen concentration before casting. Fused ferrovanadium nitride is produced from FeV40 and FeV50 alloys according to State Standard GOST 27130, and sintered ferrovanadium nitride is produced from FeV80 and FeV60 alloys. Table 2 presents the basic characteristics of fused SHS ferrovanadium nitride in comparison with NITROVAN alloy and furnace-nitrided ferrovanadium nitride corresponding to Technical Specifications TU 14-5-122–80. Table 2. Composition and properties of nitrogen-bearing vanadium alloys  Fused ferrovanadium nitride may be used in smelting HSLA steel, as well as rail steel and high-speed steel. Nitrogen assimilation is 86–98 %. Note that the nitrogen assimilation of vanadium itself is exceptionally high: more than 95 %. Thus, the new production technology for ferrovanadium nitride based on metallurgical self-propagating high-temperature synthesis yields a product of high density, which is typical of fused alloying materials, and with the maximum nitrogen content, which is typical of sintered alloying materials. Thanks to its composite structure—α Fe(Mn)–δ VN—the fused ferrovanadium nitride is very strong. This completely eliminates alloy disintegration in various operations and also prevents the formation not only of dust particles but of any chunks smaller than 10 mm. Published in "Steel In Translation" Volume 39, Number 11, 2008 (article in pdf)
Ferrovanadium nitride FERVANIT®, produced by NTPF Etalon Co., Ltd: Other publications about SHS |
