(3519) 580-155, mail@ntpf-etalon.ru
|
|
Ferrovanadium Nitride SH-SynthesisM. Kh. Ziatdinov1, I. M. Shatokhin2
1 - Tomsk State University
2 - NTPF Etalon Co., Ltd. (Magnitogorsk, Russia)
The production of high-strength low-alloy (HSLA) steel is steadily expanding on account of its economic benefits. Such steel is invaluable in industry, especially in fields such as construction, the auto industry, bridge and ship building, and railroad and pipeline manufacture. The high performance of HSLA steel results from microalloying with carbide- and nitride-forming metals (Al, Ti, V, Nb, etc.). On cooling and as a result of special rolling and heat treatment, finely disperse carbides, nitrides, and/or carbonitrides of these metals are deposited in the steel ingot. These deposits usually measure no more than tens of nm. Such nanocomponent steel is strong, plastic, and weldable and retains its operational characteristics at extremely low temperatures. HSLA steel microalloyed with vanadium and nitrogen is of particular economic importance. The vanadium nitrides and carbonitrides formed in the steel on cooling and thermomechanical treatment in the form of nano-particles facilitate pronounced reduction in grain size. The combination action of dispersional hardening and reduction in grain size improves a whole range of mechanical characteristics of the steel. In steel smelting, at present, nitrogen-bearing gases or special alloying composites are used for the microintroduction of nitrogen. In practice, both methods will often be used at once. Some of the nitrogen is absorbed by the melt directly from the gas phase, but the composition is finally corrected by means of a nitrogen-bearing alloy, either in traditional chunk form or as the filler in powder wire. From the first industrial introduction of HSLA steel with vanadium-nitride hardening in the decade 1960–1970, various alloys have been proposed for the combined introduction of vanadium and nitrogen in the steel. These alloys are obtained by solid-phase nitriding of powder or by the nitriding of melts. However, only two materials are basically used at present: ferrovanadium nitride and vanadium carbonitride, produced by a solid-phase technology. Vanadium carbonitride was developed more than 30 years ago and is currently known as NITROVAN alloy. Its attractive aspects include high-nitrogen content (up to 16 %) and production directly from vanadium oxide by reduction with carbon in vacuum furnaces. Its practical use is complicated by its high melting point (>2400 °C) and the low briquet density (~3 g/cm3). In steel smelting, this reduces the assimilation not only of nitrogen but of vanadium. Moreover, the absorption of nitrogen is unstable from melt to melt . Similar effects are observed when using nitrided ferrovanadium produced in vacuum furnaces. Furnace technology is also used in the production of ferrovanadium nitride. In that case, the raw material is standard ferrovanadium powder. The solubility of nitrogen in liquid ferrovanadium is relatively high. The alloy containing 41.5–50.6 % V dissolves 3.8–5.3 % N at 1900 °C and atmospheric pressure. The quantity of dissolved nitrogen declines with increase in temperature and decrease in pressure. However, despite the good solubility of nitrogen in ferrovanadium melt, the nitriding of liquid alloys to obtain steel additives has not been industrially adopted on account of the high viscosity of nitrogen-bearing ferrovanadium melts and the difficulty of obtaining high nitrogen concentrations in large metallurgical systems. Evidently, there is no prospect for the production of nitrogen-bearing ferrovanadium alloys by plasma smelting, because it is prohibitively expensive and the nitrogen concentration in the product is too low. Thus, the only real alternative to furnace production of nitrogen-bearing alloying materials is self-propagating high-temperature synthesis. At first, self-propagating high-temperature synthesis was employed for the production of oxygen-free inorganic refractory powders. This application is based on the highly exothermal formation of compounds such as carbides, nitrides, borides, and silicides. Later, self-propagating high-temperature synthesis was adapted for the synthesis of alloying composites and other metallurgical materials. This version of self-propagating high-temperature synthesis - sometimes called metallurgical self-propagating high-temperature synthesis - is based on exothermal oxygen-free exchange reactions using various ferroalloys as the basic raw materials. In the present work, we develop an industrial production technology for ferrovanadium nitride based on self-propagating high-temperature synthesis. Preliminary laboratory research focuses on industrial ferrovanadium alloys of different composition corresponding to State Standard GOST 27130–80 (Table 1) and metallic vanadium smelted by heating with metal in accordance with Technical Specifications TU 48-20–72 (purity 97.8% V). In addition, in studying the possibility of producing alloying composites for HSLA steel that contain vanadium nitride, we employ standard FS75 ferrosilicon (GOST 1415–78), Mn95 manganese (GOST 6008–90), and FNb60 ferroniobium (GOST 167730–85). The research method is analogous to that in [Self-Propagating High-Temperature Synthesis of Ferrosilicon Nitride, 2]. Table 1. Composition of initial ferroalloys
In the vanadium–nitrogen system, two stable nitrides are formed: δ-VN (in the region of homogeneity VN0.68-VN0.93) and β-V2N (in the region VN0.37-VN0,43). An α-solid solution of nitrogen in vanadium is also observed. In addition, in certain conditions of cooling and heat treatment, various unstable phases may be distinguished in the structure of nitrided vanadium: V16N, V13N, V9N, etc. The physicochemical and thermodynamic properties of stable vanadium nitrides vary greatly within the homogeneity region. For example, the melting point of vanadium mononitride falls from 2340 °C for VN0,98 to ~2260 °C for VN0,7. The reaction of vanadium with nitrogen is accompanied by the release of considerable heat. The thermal effect of the formation of stoichiometric vanadium nitride VN is 217.2 ± 5 kJ/mole; the corresponding figure for V2N is 270.6 ± 5.1 kJ/mole. Heat is also liberated when nitrogen dissolves in vanadium. Such exothermal nitride formation permits saturation of vanadium with nitrogen in self-sustaining combustion over a wide range of process parameters. The considerable heat liberation in the synthesis of vanadium nitrides is also the basis for the nitriding of industrial ferrovanadium alloys by self-propagating high-temperature synthesis, since the formation of iron nitrides is nonexothermal, while the nitrides are thermally unstable. Thus, research shows that, if iron powder is added to vanadium powder, the combustion rate of this mixture declines rapidly with increase in iron concentration. The nitrogen content in the product also gradually declines. The dependence of the combustion rate of ferrovanadium on the vanadium concentration is shown in Fig. 1. For comparison, we present an analogous dependence of mixtures of vanadium powder with iron. It is evident that, on switching from metallic vanadium to FeV80 and FeV60 alloys, the combustion rate declines rapidly; in this range, the combustion rate of V–Fe alloys and mixtures of V and Fe powder is similar. However, with further decrease in vanadium concentration in the alloy (on transition to ferrovanadium FeV50), the combustion rate rises sharply (more than twofold). At the same time, the increase in iron concentration in the powder mixture from 40 to 45 % leads to further monotonic decline in combustion rate. A 50:50 (by mass) mixture of vanadium and iron powder cannot be nitrided in combustion. 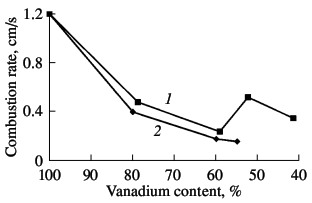 Fig. 1. Influence of the vanadium content in ferrovanadium
on the combustion rate: (1) ferrovanadium alloys; (2) mix- tures of vanadium and iron powder. According to X-ray phase data, the initial ferrovanadium alloys FeV80 and FeV60 have the crystalline structure of the α-phase (a solid solution of iron in vanadium), while ferrovanadium alloys FeV50 and FeV40 have the structure of the σ phase (the tetragonal intermetallide equiatomic compound VFe). According to the V–Fe phase diagram, in the concentration range 35–55 % V, the alloys undergo phase transformation on heating to 1100–1200 °C. The transformation of the σ-intermetallides to an α-solid solution occurs at very high speed. Sharp increase in the diffusion during and immediately after phase restructuring is associated with the unexpected acceleration of the combustion of σ ferrovanadium relative to the α-alloy. Photographs for a section of a sample in which combustion was stopped by quenching are shown in Fig. 2. Measurements of the microhardness of unnitrided particles directly adjacent to the combustion zone (a narrow layer of the sample, thickness ~0.1 mm), which is characterized by intense nitrogen absorption, reveal sharp change in their hardness. The particles closer to the combustion zone are characterized by lower microhardness (~3 kPa); this corresponds to the microhardness of α-ferrovanadium. Particles at lower temperature, some distance from the combustion zone, are characterized by higher microhardness (~14 kPa), corresponding to the initial σ-ferrovanadium.  Fig. 2. Microstructure of the combustion zone of a quenched FeV50 sample: (1) σ-phase; (2) α-phase.
Layer-by-layer X-ray phase analysis confirms the metallographic data for samples with a frozen combustion front. Thus, if the initial ferrovanadium is recorded as a σ-phase, and the final product consists of α-iron and δ-vanadium mononitride, they are separated by a very thin powder layer directly adjacent to the initial alloy. X-ray diffraction data indicate that this is an α solid solution of iron and vanadium. Thus, accelerated combustion of industrial ferrovanadium alloys with a σ-ntermetallide structure is associated with σ→α phase transition in the heating zone at the corresponding temperature. In nitriding model ferrovanadium alloys and other ferroalloys, it is found that the absorption of nitrogen usually occurs in two stages. Most of the nitrogen is absorbed by the alloy in the combustion wave during layer-by-layer nitriding; the remainder is absorbed by the nitrogen-bearing intermediate product after passage of the laminar-combustion front, as a result of final bulk reaction. Two conditions are necessary to ensure two-stage nitriding. First, within the combustion wave, the transformation of the metal (in the present case, vanadium) to nitride should be less than complete, so as toleave the possibility of bulk combustion. Second, the nitrogen-bearing intermediate product formed behind the nitriding front must retain high permeability (porosity), so that nitrogen from the surrounding atmosphere may enter the sample without obstruction and be fixed there in nitride form. In many cases, both conditions are satisfied. Two methods may be employed to observe the stages of nitrogen absorption in filtrational alloy combustion. The first is based on combustion in special equipment for self-propagating high-temperature synthesis, where the change in sample mass during the process is continuously recorded. The corresponding curves for industrial ferrovanadium are shown in Fig. 3. It is evident that the variation in sample mass over time for FeV80 and FeV60 alloys is qualitatively different from that for FeV50 and FeV40 alloys. The gravimetric curves for the σ-alloys have a point of discontinuity beyond which the sample mass remains constant. The gravimetric curves for the α-alloys (FeV80 and FeV60) have no such discontinuity. Beyond the linear section corresponding to layer-by-layer combustion, the curves smoothly tend to a maximum (complete bulk combustion). Thus, we may say that, in the nitriding of ferrovanadium alloy corresponding to the range of existence of α-phase (>55% V), two-stage absorption of nitrogen occurs. In the first stage, ~75% N is absorbed in conditions of layer-by-layer combustion; the remaining nitrogen is absorbed in α-ferrovanadium during final bulk combustion. An alloy whose vanadium content corresponds to the range of existence of σ-phase (35–55% V) will be nitrided in a single-stage process. All the nitrogen within the intermetallide alloy (vanadium mononitride) is absorbed during layer-by-layer combustion. 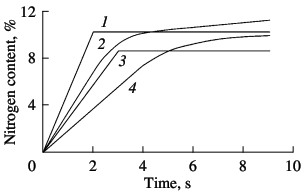 Fig. 3. Parametric nitriding curve of ferrovanadium:
(1) FeV50; (2) FeV80; (3) FeV40; (4) FeV60. The different mechanisms of nitrogen saturation for α- and σ-ferrovanadium are confirmed by layer-by-layer chemical analysis of quenched samples. By sharp cooling of burning samples, combustion is halted. The samples are then subjected to chemical analysis by the Kjeldahl method. The samples are taken from layers directly adjacent to the combustion front on the nitriding-product side (at a distance of ~0.5 mm). The results are shown in Fig. 4: 1) calculated values of the maximum possible nitrogen content with complete conversion of vanadium to stoichiometric mononitride; 2, 3) nitrogen content in the combustion products of the alloys and mixtures, respectively, with slow cooling of samples; 4) nitrogen concentration in quenched samples. Comparison of curves 2 and 4 indicates that, whereas the nitrogen concentration in quenched samples is markedly less than that in slowly cooled samples for vanadium and α-ferrovanadium (alloys with 78.8 and 59.2% V), the corresponding nitrogen concentrations are practically the same for σ-ferrovanadium (alloys with 52.4 and 41.6 % V). This confirms the conclusion derived from experiments with continuous weighing of samples during nitriding. In the combustion of alloys corresponding to the σ-intermetallides, nitrogen is absorbed in a single stage during layer-by-layer combustion. If ferrovanadium with the structure of an α-solid solution is nitrided, two-stage absorption is observed. Nevertheless, the conversion of vanadium to nitride is greater in σ-ferrovanadium than in α-ferrovanadium: 83 and 84 % for FeV50 and FeV40 alloys; and 68 and 79 % for FeV80 and FeV60 alloys. Formetallic vanadium, the corresponding figure is 58 %.  Fig. 4. Influence of vanadium content in ferrovanadium on
the degree of nitriding. As for other ferroalloys and metals, the nitriding of ferrovanadium at pressures up to 15 MPa occurs as filtrational combustion. The distinguishing feature of such self-propagating high-temperature synthesis is a strong dependence of the combustion characteristics and the composition of the reaction products on the nitrogen pressure, the fineness of the initial powder, and the permeability of the powder batch. The corresponding curves of FeV80, FeV60, FeV50, and FeV40 alloys are shown in Figs. 5–7. In all cases, increasing the nitrogen pressure, reducing the particle size of the ini-tial alloys, and reducing the porosity of the nitrided samples boosts the combustion rate. The quantity of nitrogen in the reaction products is higher on nitriding coarser powder, when using high nitrogen pressure, and when using more permeable samples. These results are expected and qualitatively agree with the data obtained in the combustion of model alloys similar in composition to the industrial alloys. Analogous results were obtained earlier for the nitriding of metal and ferroalloy powders. 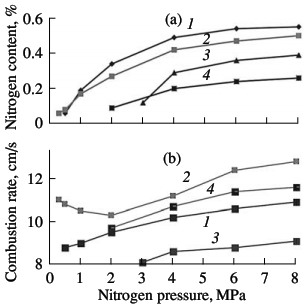 Fig. 5. Influence of the nitrogen pressure on the combustion rate (a) and degree of nitriding (b) of ferrovanadium: (1–4) as in Fig. 3
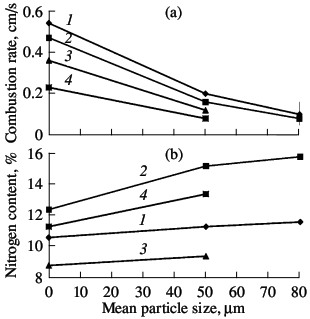 Fig. 6. Influence of the size of the powder particles on the
combustion rate (a) and degree of nitriding (b) of ferrovanadium: (1–4) as in Fig. 3 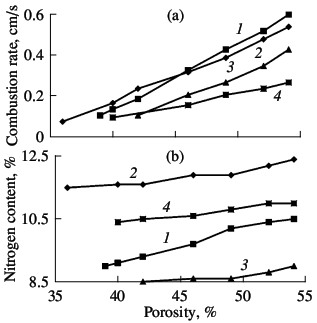 Fig. 7. Influence of the sample porosity on the combustion rate (a) and degree of nitriding (b) of ferrovanadium: (1–4) as in Fig. 3
The combustion temperature in nitriding ferrovanadium is measured by means of VR5/VR20 tungsten–rhenium thermocouples. Typical temperature profiles for α- and σ-ferrovanadium are shown in Fig. 8. The temperature in the combustion wave of the intermetallides alloy grows very rapidly and then remains constant for a long time. The combustion temperature of α-ferrovanadium is markedly less, but the final temperature is higher. The maximum combustion temperature varies quite widely, depending on the nitriding conditions: 1780–2060 °C for FeV80; 1630–1830 °C for FeV60; 1480–1560 °C for FeV50; and 1420–1490 °C for FeV40. Analysis of the results shows that, in general, the maximum temperature correlates well with the nitrogen content in the product: if more nitrogen is absorbed by the alloy, there will be more heating on combustion. The qualitatively different temperature profiles indicate different combustion mechanisms of the α- and σ-ferrovanadium: single-stage nitrogen absorption by the intermetallide alloy and two-stage absorption by the solid-solution alloy. 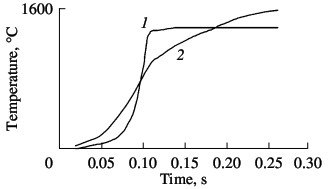 Fig. 8. Temperature profiles of ferrovanadium combustion:
(1) σ-FeV; (2) α-FeV. We know that the economic efficiency of vanadium increases when certain vanadium alloys are used, including nitrogen-bearing alloys. In the present work, we investigate the possibility of obtaining complex nitrided alloys that contain vanadium as well as manganese, niobium, and silicon. These components are obtained from powders of standard industrial alloys: ferroniobium, ferromanganese, and ferrosilicon. Although the heat of formation of nitrides of silicon (Si3N4), niobium (NbN), and manganese (Mn3N2) are completely comparable with those of vanadium nitrides, their reaction with nitrogen is much less intense. As we see in Fig. 9, all three additives reduce the combustion rate of ferrovanadium. Manganese and niobium somewhat reduce the nitrogen concentration in the reaction products; ferrosilicon, by contrast, considerably increases the nitrogen concentration. Thus, self-propagating high-temperature synthesis permits the production of any complex alloying composite containing V, Nb, Mn, and/or Si on the basis of the corresponding standard-ferroalloy powder. The nitrogenconcentration is close to the maximum for the chosen composition. 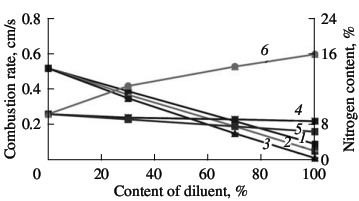 Fig. 9. Influence of the ferroalloy dilution on the combustion rate (1–3) and degree of nitriding (4–6) of ferrovanadium: (1, 4) FeNb; (2, 5) Mn; (3, 6) FeSi.
Laboratory data for industrial ferrovanadium alloys permit the development of a production technology for ferrovanadium nitride on large equipment (working volume 0.15 m3). The combustion rate in such industrial reactors for self-propagating high-temperature synthesis is determined by means of a flow-rate meter mounted at the inlet. After combustion is initiated by sending an electric pulse to the ignition unit, the nitrogen flow rate sharply rises, and quickly reaches a constant value. Nitriding at constant flow rate corresponds to steady layer-by-layer combustion. The flow-rate meter records the end of this stage from the sharp change in the rate of nitrogen absorption. In nitriding σ-ferrovanadium, the nitrogen flow rate falls practically to zero, as confirmed by the absence of final bulk reaction. In the nitriding of α-ferrovanadium, after rapid decline in the nitrogen absorption rate recorded by the flow-rate meter, saturation of the alloy with nitrogen continues at a lower and constantly decreasing rate. The combustion rates of ferrovanadium measured in this way in the industrial reactor are close to those obtained in the laboratory. Thus, the combustion of FeV50 alloy at 12 MPa lasts 5–8 min, which corresponds to a rate of layer-by-layer combustion of 0.2–0.3 cm/s. The absorption rate of nitrogen by ferrovanadium is ~0.1 m3/s. The nitrogen concentration in the combustion products of industrial ferrovanadium depends on the initial alloy, the nitrogen pressure in the reactor during combustion, the fineness of the powder, and various other factors and may vary within the range 8–16 %. The traditional method is employed to attain the maximum possible (limiting) nitrogen content in the combustion products. To reduce the exothermal character of the batch, reaction products are usually added. This reduces the temperature of the process, on the one hand, and, on the other, prevents particle coagulation at the combustion front and reduces the permeability behind the front, thereby creating conditions for maximum conversion of the metal to nitride in the final nitriding stage. The influence of dilution with reaction products is investigated for the combustion of FeV80 and FeV60 ferrovanadium. The nitrogen content in the combustion product is plotted in Fig. 10 as a function of the quantity of nitriding products in the initial mixture. The particle size is the same in all the powders (0.04 mm). FeVN80 ferrovanadium nitride powder synthesized from FeV80 alloy contains 12.1 % N; FeVN60 ferrovanadium nitride from FeV60 alloy contains 10.9 % N. It is evident from Fig. 10 that the limiting nitrogen concentration in FeV80 corresponds to the addition of 30 % FeVN80 to the batch, while the limiting nitrogen concentration in FeV60 corresponds to the addition of 15 % FeVN60 to the batch. The combustion rate of mixtures diluted with the final product declines rapidly with increase in diluent concentration. 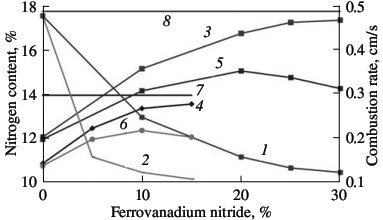 Fig. 10. Influence of the ferroalloy dilution on the combustion rate (1, 2) and degree of nitriding (3–6) of ferrovanadium: (1, 3, 5) FeV80 (FeVN80); (2, 4, 6) FeV60 (FeVN60); (3, 4) total nitrogen content; (5, 6) increment in nitrogen on combustion; (7, 8) limiting nitrogen content for FeV60 (7) and FeV80 (8).
Two types of ferrovanadium nitride produced by self-propagating high-temperature synthesis (SHS) have been developed for industrial use: fused and sintered ferrovanadium nitride. Fused SHS ferrovanadium nitride is used for traditional chunk alloying of steel in the ladle or directly in the furnace. Sintered SHS ferrovanadium nitride serves as an effective filler for powder wire in adjusting the nitrogen concentration before casting. Fused ferrovanadium nitride is produced from FeV40 and FeV50 alloys according to State Standard GOST 27130, and sintered ferrovanadium nitride is produced from FeV80 and FeV60 alloys. Table 2 presents the basic characteristics of fused SHS ferrovanadium nitride in comparison with NITROVAN alloy and furnace-nitrided ferrovanadium nitride corresponding to Technical Specifications TU 14-5-122–80. Table 2. Composition and properties of nitrogen-bearing vanadium alloys
Fused ferrovanadium nitride may be used in smelting HSLA steel, as well as rail steel and high-speed steel. Nitrogen assimilation is 86–98 %. Note that the nitrogen assimilation of vanadium itself is exceptionally high: more than 95 %. Thus, the new production technology for ferrovanadium nitride based on metallurgical self-propagating high-temperature synthesis yields a product of high density, which is typical of fused alloying materials, and with the maximum nitrogen content, which is typical of sintered alloying materials. Thanks to its composite structure—α Fe(Mn)–δ VN—the fused ferrovanadium nitride is very strong. This completely eliminates alloy disintegration in various operations and also prevents the formation not only of dust particles but of any chunks smaller than 10 mm. Published in "Steel In Translation" Volume 39, Number 11, 2008 (article in pdf)
Ferrovanadium nitride FERVANIT®, produced by NTPF Etalon Co., Ltd: Other publications about SHS |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
