- Главная
- Продукция ∇
- Композиционные легирующие материалы
- Огнеупорные материалы
- Бориды металлов
- Услуги по сварке
- Комплекс по переработке отходов металлургии
- Примеры реализованных нестандартных проектов
- Оборудование для установок вакуумирования стали
- Горелочные устройства
- Фурмы
- Системы газоочистки
- Оборудование для электросталеплавильных печей
- Производство теплообменного оборудования
- Воздушные теплогенераторы
- Вибрационные мельницы
- Дробилки щековые ДЩ
- Барабанные сушила
- Гидравлические маслостанции
- Грузоподъемное оборудование
- Емкости и резервуары
- Дисковые компенсаторы
- Строительные металлоконструкции
- Минеральная вода
- Услуги по дроблению и помолу материалов
- Услуги гидроабразивной и плазменной резки металла
- Азот
- Сертификация
- Публикации
- Контакты
Общество с ограниченной ответственностью
"Научно-техническая производственная фирма "Эталон"
455030, Россия, Магнитогорск, Западное шоссе, 15
(3519) 580-155, mail@ntpf-etalon.ru
(3519) 580-155, mail@ntpf-etalon.ru
|
|
SHS-technology of ferroalloys nitridingM. Kh. Ziatdinov1, I. M. Shatokhin2
1Tomsk State University
2NTPF Etalon Ltd Abstract Nitrogen-containing alloys of manganese, chromium, vanadium and silicon are used for melting various grades of nitrogen alloyed steels. Such addition alloys are traditionally obtained by means of high-temperature processing of green alloys powder in vacuum furnaces in nitrogen atmosphere. This paper deals with the results of the design and commercial development of an alternative technology for the production of nitrogen-containing ferroalloys based on principies of self-propagating high-temperature synthesis (SHS). The formation of manganese nitrides (Mn4N, Mn2N), chromium nitrides (Cr2N, CrN), vanadium nitrides (V2N, VN), and silicon nitride(Si3N4) can be attended by a great release of heat. Such heat release enables to organize the nitriding process in self-sustained combustion. This process similarly to metallothermic combustion proceeds without an electric furnace being used, and it is carried out in special units, i.e. SHS reactors. SHS units are equipped with ignition and cooling systems and can withstand high pressures of up to 15 MPa. Powder ferroalloys are exposed to nitriding by being placed in nitrogen atmosphere. The local heating of powder surface layer is effect by feeding an electric pulse, which initiates exothermic reaction of nitrides formation in it. The released heat warms the next layer of source mixture in which the reaction of nitride formation is also activated. Then, layer by layer, the whole powder is getting nitrided. The velocity of wave propagation in the course of such layer-by-layer nitriding (combustion rate), the temperature resulted from heat release (combustion temperature), and the nitrogen content of the product depend on the nitrogen pressure, the composition of the green powder, its dispersion, and a number of other parameters. By selecting the optimum combination of the above-mentioned parameters for each alloy, there have been designed processing methods for the production of addition alloys with the best combination of properties, such as nitrogen content, density and volume distribution of nitrogen. Introduction To date nitrogen has been used as an alloying element for over 70 years. The first results of the testing of nitrogen alloying were published in the 1930s. Due to the acute shortage of nickel topical for the time, there had appeared an urgent need to substitute it in stainless steels. Nitrogen alloying turned out to be one of the successful way-outs. First of all, it was possible to use the property of nitrogen to stabilize austenite as, thanks to its stabilizing capacity, 0.2 % of nitrogen is equivalent to ~4 % of nickel. Moreover, nitrogen as opposed to carbon (also being an austenite stabilizer) does not reduce the corrosion resistance of steel. Later, nitrogen began to be used for the dispersion strengthening of low-alloyed steels. The most essential advantage of nitrogen as an alloying element is its availability and its being almost unlimited as an natural resource. Nitrogen recovery does not affect the environment or produce any waste. As nitrogen only occurs in nature in the form of gas, in order to introduce it into steel it is necessary to turn nitrogen into a solid matter, and such a nitrogen-bearing material must be compatible with steel melt. Despite the fact that there are new technologies of the direct introduction of gaseous nitrogen into a liquid metal which are successfully developing now, alloying with the use of solid nitrogen sources still remains the principal method of nitrogen-bearing steel melting due to the possibility of applying this method to various grades of steel. A new technology of introducing nitrogen into steel by using flux cored wire with high-nitrogenous compounds inside has triggered new applications of solid nitrogen bearers. Nitriding metals, ferroalloys or nitrogen-bearing chemical compounds serve as nitrogen sources. The choice of this or that alloying material is mainly determined by the composition of the metal being melted and the technology of its production. At this point, it is compatible with the steel composition, and the nitrogen concentration in it is just enough for the frugal introduction of its required quantity into steel. Historically, the first sources of nitrogen were chrome-based alloys, because first produced nitrogen-bearing steels were stainless chrome alloys. Then, manganese-based nitrogen-bearing rich alloys appeared as a response to the development of nitrogen-bearing Cr-Mn-steels. Due to the development of low-alloyed high-strength steels with carbonitride strengthening, the market began to offer high-nitrogenous vanadium rich alloys. As the production volume of nitrogen-bearing steels was increasing and their range was expanding, the assortment of alloying materials for the maintaining of the elevated nitrogen concentration also changed. Along with the enhancement of the composition and structure of nitrogen-bearing rich alloys on bases which had already become classic (Cr, Mn, V), nitrogen-bearing compounds from other industries began to be used. For example, the high-nitrogenous compound CaCN2 (~23 % N) was borrowed from chemistry although it had originally been intended to be used as a herbicide in agriculture. The ceramic compound silicon nitride Si3N4 (~39 % N) came from the refractory industry. Another high-melting compound, vanadium carbonitride (VCN), has been used for alloying of HSLA-steels for many years already. The long-term experience of the use of nitrided rich alloys has enabled to work out certain requirements for their quality. First of all, alloys for steel nitriding are to have the maximum possible content of nitrogen, which is necessary to achieve their minimum discharge. Secondly, the base of such nitrogen-bearing rich alloys is to be compatible with the grade of the metal being melted. Besides, alloying nitrogen alloys are to dissolve in steel melt as fast as possible so as not to increase the heat time, and the nitrogen fixation is to be high and stable to the limit. In the nowadays metallurgy it is typical for a steel composition to be specified with the lowest possible concentration of alloying elements, particularly nitrogen. The production materials and alloying technology are chosen with a view to finding the most economical way of achieving the required content of nitrogen and other alloying elements in the metal. Let us have a closer look at the technology of making nitrogen-bearing rich alloys as exemplified by ferrosilicon nitride synthesis. Silicon nitride is one of the few oxygen-free refractory compounds; it is widely used in industry on account of its unique physicochemical characteristics. The largest consumption volume for silicon nitride and materials based on it lies in the refractories industry. Refractory components for lining blast furnaces are common, as are those for lining aluminum electrolyzers, furnace devices and other high-temperature plants. Another area of large-scale use for silicon nitride materials is in the production of unshaped refractories: tap-hole and trough parts for blast-furnace use. Numerous researches and tests have shown that silicon nitride is an irreplaceable component of the latest refractory materials. In the 1970s, a new refractory known as ferrosilicon nitride came on the market; it is produced from powder with a high silicon content that is saturated with nitrogen in a high-temperature resistance furnace. The ferroalloy nitrided in this way consists mainly (70-80 %) of silicon nitride Si3N4, together with iron and/or its silicides and impurities typical of standard ferrosilicon with 75 % Si. Ferrosilicon nitride is intended for use as a strengthener in unshaped refractory mixtures. Tap-hole and ranner materials for blast-furnace production made with the addition of ferrosilicon nitride proved highly effective and are widely used. Somewhat later, ferrosilicon nitride began to be used for alloying in smelting corrosion-resistant steel with an elevated nitrogen content by electroslag remelting under pressure. Thanks to the high nitrogen content, a ferrosilicon nitride used for alloying and its relatively low carbon content, it is possible to smelt high-strength steel with the maximum quantity of nitrogen (more than 1.0 %). The consumption of alloying material is minimal here, with relatively high nitrogen assimilation by the melt. In comparison with the traditional manganese and chromium alloys used for alloying, the consumption of the ferrosilicon nitride is considerably less. The traditional furnace technology for making ferrosilicon nitride is similar to that for nitriding pure silicon and consists in high-temperature processing for many hours of the initial powder in a nitrogen atmosphere. Nitriding silicon and ferrosilicon is also much influenced by their distinctive properties such as highly exothermic reaction, relatively low melting point of silicon (1415 °C) and particularly of ferrosilicon (about 1200 °C), in addition to the comparatively low thermal stability limit for silicon nitride (about 1900 °C at 0.1 MPa). The thermal stability of the nitride is also reduced in contact with molten iron. Therefore, the saturation of silicon or its alloys with nitrogen under industrial conditions is conducted in steps. In the initial stage, at a temperature below the melting point of silicon (ferrosilicon), one has solid-state nitriding, which produces a form of nitride barrier framework. The final nitriding is performed at a higher temperature. Furnace ferrosilicon nitride usually contains around 30% N. The same material is used for alloying steel with nitrogen and for strengthening unshaped refractories. Ferrosilicon nitride sythesis A highly exothermic formation of silicon nitride provides a basis for nitriding in a self-maintaining state of burning. Self-propagating high-temperature synthesis (SHS) in relation to the synthesis of pure silicon nitride has been researched in fair detail. Nitriding of ferrosilicon alloys is appreciably less exothermic, since the formation of iron nitrides (Fe4N, Fe2N) involves hardly any heat effect. The reduction of heat release in nitriding ferrosilicon will also be favored by the fact that the silicon and iron are linked as thermodynamically stable silicides, so on burning part of the heat is consumed in decomposing them. Research on nitriding in the Si-N2 system has shown that there is a difference from the burning of metals groups IV- V of the periodic system in that the synthesis of Si3N4can be realized only by using very finely divided silicon powder, preferably of submicron size, because silicon nitride belongs to the class of covalent nitrides, which have virtually no homogeneity regions and low diffusion mobilities for the Si and N atoms. Also, the system has very low solubility of nitrogen in liquid or solid silicon. The SHS ferrosilicon nitride is based on silicon nitride, which is a source of nitrogen when using the material as an alloying additive. If ferrosilicon nitride is introduced in refractories, the Si3N4 has a strengthening effect. Pure silicon nitride contains almost 40 % nitrogen (the stoichiometric content is 39.94 % N). It is practically inhomogeneous. At normal pressure (0.1 MPa), silicon nitride breaks down without melting at ~1900 °C. Raising the pressure increases the temperature stability of Si3N4. On contact with steel melt, the silicon nitride actively reacts, releasing nitrogen. Combustion teperature The formation of silicon nitride is accompanied by the liberation of large quantities of heat: 3Si + 2N2 -> Si3N4 + 751,8 kJ/mol
Because the reaction is highly exothermal, the synthesis of silicon nitride is possible in self-sustaining combustion. The theoretical adiabatic temperature of silicon combustion in nitrogen is exceptionally high-more than 4000 °C. However, this corresponds to the assumptions that all the silicon is converted to nitride and that there are no heat losses to the surroundings. It is also assumed that the products of synthesis do not sublimate. In practice, these conditions are very difficult to reproduce. Therefore, the experimental maximum temperature when silicon burns in nitrogen is much less: 1900-2200 °C. The adiabatic temperature of ferrosilicon combustion in nitrogen will be lower when the theoretical combustion temperature of silicon is lower, since the thermal effect of the reaction between the alloy and nitrogen is known to be less than the exothermal effect of the pure metal. This is mainly because the ferrosilicon contains a considerable quantity of iron, which, in contrast with silicon, reacts with the nitrogen practically without heat liberation, while the nitrides formed here are thermally unstable. Another factor is that silicon and iron are bound in thermally stable silicides, whose decomposition requires considerable energy consumption. The combustion temperature is calculated from the conditions of equal enthalpies of the initial materials at the initial temperature (T0) and the products of synthesis at the combustion temperature (Tc). Thus, all the heat liberated in Si3N4 formation is consumed in heating of the products, i.e., silicon nitride and iron: μ[H(Tc) - H(T0)]Si3N4 + (1 - μ)[H(Tc)-H(T0)]Fe = μQ,
where Q is the thermal effect of Si3N4 formation; is the proportion of silicon nitride in the product; H(Tc), H(T0 are the enthalpies of the combustion products at T0 and Tc. The combustion temperature calculated by this formula is relatively high for alloys with different Si content. Thus, even for ferrosilicon with 45 % Si, the theoretical combustion temperature is ~2500 °C. Hence, certain thermodynamic preconditions must be satisfied for successful self-propagating high-temperature synthesis in the ferrosilicon-nitrogen system over a broad range of initial alloy composition. In fact, experiments confirm that combustion may occur for all ferroalloys containing more than 40 % Si. Filtration Combustion In many ways, the combustion of ferrosilicon in nitrogen is similar to the combustion of metallic silicon. Thus, in nitriding, it is found that the combustion products contain considerable unreacted silicon, and consequently the combustion temperature is relatively low. Such incomplete conversion of silicon to the nitride is due to the low melting point of the silicon itself (1415 °C) and the relatively low dissociation temperature of its nitride. The conversion of silicon to the nitride in the experiments is 50-60 %. The melting point of Fe-Si alloy is even lower than for silicon. In alloys with 40-80 % Si, the liquid phase is formed above 1210 °C. Hence, processes associated with melting of the initial material in ferrosilicon combustion may be more pronounced. We know that a filtration combustion mechanism is responsible for the nitriding of metal powders, including silicon. In the filtration version of self-propagating high-temperature synthesis, the gaseous reagent is supplied to the combustion zone by filtration through a porous medium formed by the metallic component. In nitriding ferrosilicon, filtration is maintained by the difference in nitrogen pressure between the reaction zone and the surrounding. The pressure in the combustion zone falls steadily as a result of nitrogen absorption by the alloy at high temperature. Nitrogen must be supplied to the combustion zone by filtration from outside because, in ordinary conditions, the quantity of nitrogen in the pores of the ferrosilicon powder is very small and clearly insufficient to maintain nitriding by self-sustaining combustion. Moreover, calculations show that the nitrogen in the pores is insufficient for nitriding of the ferrosilicon even at elevated pressure. For example, at 10 MPa, the nitrogen within the powder is only sufficient to bind 1-5 % Si in the nitride, depending on the ferrosilicon composition and the porosity of the powder batch. That degree of nitriding may increase the temperature in the Fe-Si-N2 system by no more than 200-500 °C, which not only is insufficient for a self-propagating process but also eliminates the possibility of reaction. For filtration combustion, the process parameters depend strongly on the characteristics of the reacting system that determine the filtration conditions: specifically, the nitrogen pressure, the dispersity of the ferrosilicon powder, and the porosity and geometric dimensions of the batch. In addition, the parameters of ferrosilicon combustion in nitrogen depend on the initial conditions determining the thermal balance in the system: in particular, the quantity of inert components, the initial temperature of the ingredients, the introduction of activating additives, and the possibility of melting of the initial powders and/or reaction products The influence of the silicon concentration in the initial ferrosilicon and the nitrogen pressure on the combustion rate, the degree of nitriding of the alloy, and the maximum temperature in the reaction wave is illustrated in Fig. 1. 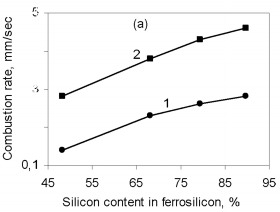 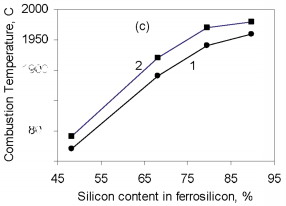 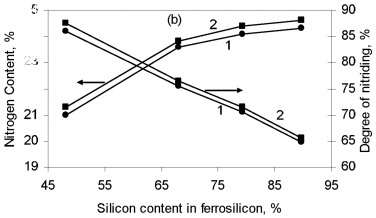 Figure 1. Influence of the silicon content in ferrosilicon on the combustion rate (a), degree of nitriding (b), and combustion temperature (c) when PN2=3 (1) and 7 MPa (2)
The results are obtained for self-propagating high-temperature synthesis in 15-L experimental apparatus. A quartz-glass window permits visual observation of the combustion process and video recording of the combustion wave propagation. The laboratory apparatus also includes a system for continuous measurement of the combustion temperature by means of thermocouples and computer recording of the temperature profile. Nitriding is based on FS90, FS75, FS65, and FS45 ferrosilicon powder with 89.9, 79.4, 68.1, and 48.25 % Si, respectively, and 0.06, 0.08, 0.07, and 0.23 % C, as well as standard quantities of other impurities. In all cases, the particle size of the powders is no more than 0.08 mm. According to the Fe-Si phase diagram, alloys with a high Si concentration consist of two phases: silicon and iron disilicide FeSi2. With increase in Si content in the alloy, the content of free silicon also increases, as confirmed by X-ray phase analysis of the initial ferrosilicon powder. Thus, whereas FS65 alloy contains no more than 25 % free silicon, FS90 alloy contains 80-85 % free silicon. Low-silicon FS45 also has a two-phase structure: about 70 % FeSi2, and the remainder FeSi. As expected, increase in the initial content of silicon is accompanied by more vigorous reaction of the silicon with nitrogen; this corresponds to considerable increase in combustion rate and increase in nitrogen concentration in the products. At the same time, the degree of nitriding of silicon in the alloys declines with decrease in their iron content. Thus, despite the relatively low nitrogen content in FS45 ferrosilicon, the degree of silicon conversion to the nitride is a maximum: almost 90 %, as against 65 % for the alloy with 90 % Si. The dependence of the combustion rate, nitrogen content, degree of nitriding, and combustion temperature on the silicon content in the ferrosilicon does not depend on the nitrogen pressure. Increase in the pressure strongly affects the combustion rate, but hardly changes the degree of nitriding of the alloys. With increase in pressure, the combustion temperature rises somewhat. Figure 2 shows the ferrosilicon burning rate and temperature as functions of the nitrogen pressure, and also the effect of the nitrogen pressure on the nitrogen content in the burning products and the extent of silicon conversion to the nitride. As would be expected, the nitrogen pressure has a large effect on the ferrosilicon burning rate. Nitrogen deficiency in the pores means that infiltration from the surrounding volume is the rate-limiting step. Improving nitrogen access to the combustion zone by raising the pressure difference intensifies the exothermic reaction. Increasing the heat production rate leads to an increase in the burning rate. The ferrosilicon burning temperature also increases with the pressure. The maximum temperature rise in the combustion zone is determined by the extent to which the silicon is converted to nitride directly in the combustion wave and by the thermophysical parameters of the medium, which determine the rate of heat removal from the combustion zone. 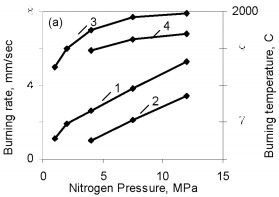 Figure 2. Effects of nitrogen pressure on (a) ferrosilicon burning rate (1 and 2) and burning temperature (3 and 4); (b) nitrogen content in product
One might assume that all the silicon in these ferrosilicon specimens is converted to stoichiometric Si3N4, and then the combustion products should contain respectively 34.5 and 31.2 % N. However, Fig. 2 shows that in the pressure range 1.0 - 12 MPa used, the degree of nitriding of the two alloys is far from maximal. The same phenomenon has been found previously in nitriding pure silicon powder. In that case, the structure of the combustion products indicated that the reason for the incomplete combustion was silicon melting. The burning temperature of ferrosilicon in nitrogen is much above the melting point. Ferrosilicons FS65 and FS75 begin to melt at about 1200 °C and at 1300 - 1350 °C the alloys are completely in the liquid state. The microstructure of burned ferrosilicon specimens confirms, that in the same way as for nitriding pure silicon, the combustion wave produces extensive coagulation of the molten ferrosilicon particles. This reduces the reacting surface, and that ultimately leads to incomplete conversion of the silicon to the nitride. The high temperature in the combustion wave favors melting of the initial ferrosilicon particles: throughout the pressure range used, that temperature greatly exceeds the melting point of the alloy. Although the Si3N4 formation reaction is highly exothermic (the calculated adiabatic burning temperature is more than 4000 °C), the burning rate of silicon in nitrogen is very low. Even when submicron powders are used, it is much less than the burning rates for thoroughly researched transition metals of groups IV-V in the periodic system. Nitriding ferrosilicon is also very slow. Even at the maximum pressure (12 MPa), the ferrosilicon burning rate only attains 0.55 - 0.60 mm/sec. Under the same conditions, the burning rate is larger by an order of magnitude in the less exothermic system FeV-N2, the reasons being the minimal solubility of nitrogen in solid or liquid silicon, the low diffusion mobility of the nitrogen and silicon atoms, and also the low thermal conductivities of silicon nitride and the initial ferrosilicon by comparison with metals and metal-like nitrides. As would be expected the ferrosilicon burning rate decreases sharply as the particle size increases. The same tendency has been observed in nitriding other metals and ferrous alloys. However, there is a difference from ferrous alloys based on transition metals of groups IV-V (ferrotitanium, ferrovanadium, and so on), whose powders burn even with a grain size of several hundred microns, whereas the burning of narrow-fraction ferrosilicon becomes impossible even at a mean particle size of 0.05 mm (Fig. 3). The nitrogen contents of the products fall as the particle size increases (correspondingly there is a reduction in the degree of conversion of the silicon to the nitride) because of the incomplete combustion of the ferrosilicon. The burning temperature of the coarser-grained ferrosilicon is also reduced. 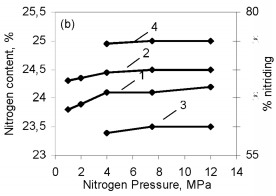 Figure 3. Effects of ferrosilicon particle size on burning rate (1 and 2) and nitrogen contents in the combustion products (3 and 4); 1 and 2: FS75; 3 and 4: FS65
The pores in the ferrosilicon powder contain too little nitrogen, and the basic quantity enters the combustion wave from the surrounding volume by infiltration. The nitrogen infiltration arises and is maintained by the pressure difference between the reaction zone and the outside, since the chemical reaction absorbs the nitrogen continuously and the pressure falls. The burning zone thus acts as a pump, which works on chemical reactions and pumps the gas into the nitride formation zone. The porosity of the ferrosilicon powder without additional forced consolidation is 60 - 70 %. It may attain 80 % for submicron powders. However, calculations show that even with this porosity the complete conversion of silicon to the nitride is possible only with a nitrogen pressure within the pores in the reaction of about 80 - 100 MPa. Consequently, one influence the infiltration of the gas into the reaction zone. Figure 4 shows that the porosity greatly influences the burning wave propagation speed. In general, the higher the porosity, the more effective the infiltration, and the better the reaction conditions, so the burning rate increases with the porosity. Reducing the porosity gradually retards the process and even layerwise burning becomes impossible. The burning temperature and extent of silicon conversion to the nitride are only slightly dependent on the porosity. One notes that increased charge density provides stronger products with elevated density. 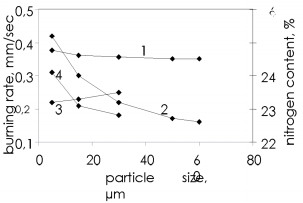 Figure 4. Effect of ferrosilicon sample porosity on the burning rate (1) and the nitrogen content of made products (2)
The infiltration mechanism for one of the components entering the reaction zone imposes certain features on the burning wave propagation for specimens with various sizes. There is a difference from gas-free burning, in which the reaction wave propagation speed is almost independent of the specimen dimensions, in that infiltration burning shows a considerable effect from the geometrical parameters because when infiltration difficulties arise (low pressure, reduced porosity), layerwise burning may go over to surface reaction. Figure 5 shows this, from the dependence of the ferrosilicon burning rate at low and high pressures on the diameter of the cylindrical specimens. When there is no effect from the specimen diameter, this implies layerwise burning wave propagation. With surface burning, the burning rate decreases as the specimen diameter increases because in surface burning the burning front initially propagates in the outer layer of the specimen. The thickness of that layer is dependent on the density and the nitrogen pressure: the higher the pressure and the greater the porosity, the greater the burning front penetration depth. The inner part of the specimen remains unreacted in the initial stage and serves as a sink for the heat, which reduces the burning rate. Although at the start the reaction wave propagates only at the surface, subsequently the nitriding develops in the central parts because the burning wave propagates from the surface. Consequently, the degree of nitriding for specimens that burn from the surface attains quite high values. 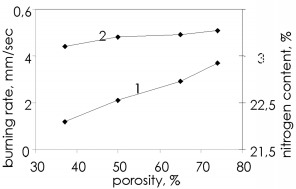 Figure 5. Effects of specimen diameter on the ferrosilicon burning rate (1 and 2): 1) pressure 1MPa 2) pressure 8MPa
The Phase Composition and The Structure Of The Products The phase compositions of the ferrosilicon nitriding products are very much dependent on the extent of silicon conversion to the nitride. With maximal conversion, the product consists of two phases β-Si3N4 and α-Fe. The amount of silicon nitride decreases as the nitrogen concentration falls. Wth a low concentration of residual silicon, the product contains mainly silicides Fe3Si and FeSi. Wth high degrees of incomplete combustion, there are considerable amounts of FeSi2 and Si; free iron almost vanishes. One can thus regulate the burning conditions to obtain a composite product based on silicon nitride (60 - 85 %). Wth maximal nitriding, the bonding agent in the composite will be iron, while with low nitrogen concentrations, one has instead iron silicides somewhat X-ray phase analysis of the combustion products of ferrosilicon in nitrogen shows that, over the whole range of initial parameters, the basic phase is β-silicon nitride. No pronounced quantities of α-silicon nitride are seen. This is probably because the α-structure is only stable up to ~1400 °C and is converted irreversibly to the β-structure at higher temperatures. The combustion temperature of ferrosilicon in nitrogen is more than 1750 °C for all the initial conditions considered, and therefore a-Si3N4 formation is unlikely. Typical X-ray patterns of the nitriding products of FS45 and FS75 are shown in Fig. 6 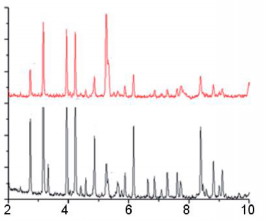 Figure 6. Typical X-ray patterns of the nitriding products of FS45 (a) and FS75 (b)
Analysis of the sample structure shows that, in all cases, considerable permeability of the ferrosilicon nitride persists. The porosity of the combustion products is 35-55 %, depending on the conditions of synthesis. Wth such high porosity behind the combustion front, intense absorption of nitrogen is possible. To determine the contribution of such nitriding to the total nitrogen content in the combustion products, combustion is interrupted by sharply reducing the pressure in the working volume of the reactor, and then introducing argon. Chemical analysis of the samples quenched in this way shows that the final combustion stage considerably increases the nitrogen content. The proportion of nitrogen absorbed after passage of the laminar-combustion wave as a result of the final combustion stage may amount to 30 %. The great contribution of the final nitriding stage to the total nitrogen content may be attributed, in particular, to the weak pressure dependence of its content in the product. The typical macrostructure of ferrosilicon nitride is shown in Fig.7. Exceptionally uniform distribution of nitrogen over the volume of the product is important here. In fact, ferrosilicon nitride is a composite consisting primarily of silicon nitride. The binder is iron and its silicides. Wth the high nitrogen content in the product, the proportion of free iron rises; with decrease in the degree of nitriding, the quantity of silicides increases. Since the density of Si3N4 (3.19 g/cm3) is much less than that of iron and its silicides, the content of silicon nitride in the product is very high (80-95 vol. %). Figure 6 shows a typical heterogeneous microstructure for the new material. Much of the material is occupied by silicon nitride particles, where the binding agent consists of iron silicides. That structure provides good performance for use in refractories.  Figure 7: Macrostructure of SHS ferrosilicon nitride
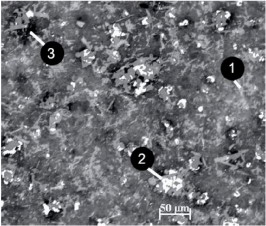 Figure 8: Typical microstructure of SHS ferrosilicon nitride: 1) silicon nitride; 2) iron silicides; 3) pores
The industrial production A 0.15-m3 SHS reactor has been developed at NTPF Etalon Ltd. for the industrial production of SHS ferrosilicon nitride and other materials. It takes the form of a thick-walled metal vessel with systems for cooling, gas injection and extraction, ignition, and sealing. Thanks to a reactor design with an accelerated-cooling system, SHS technology went into operation on an industrial scale; the working temperature is up to 2200 °C, with one-time loading of up to 0.3 t of batch. The new sealing system permits safe and reliable synthesis in filtration combustion at a nitrogen pressure up to 15 MPa. The time required to open and seal the equipment is reduced to a minimum. The shop for the production of ferrosilicon nitride and other SHS materials includes sections for crushing and fine grinding, drying and charging of the crucibles, and synthesis, as well as dispatcher and central control panels, a laboratory for intake monitoring of the raw materials, and a store for the initial materials and the final products. In the synthesis section, there are 20 SHS reactors; the total area of the shop is ~3000 m2. The new shop can produce up to 10 t of various materials per day. The universal design of the SHS reactors permits the production of a wide range of materials based on inorganic refractory compounds of nitrides, borides, carbides, sulfides, and so on. Synthesis in reactive gas (nitrogen), argon, or vacuum is possible. Relatively large-scale production (in amounts of a few tons) based on self-propagating high-temperature synthesis was introduced. In self-propagating high-temperature synthesis, besides such benefits as the absence of power consumption, the speed of the process at high pressure and maximum temperature, and the simplicity of the equipment, a new approach to the selection of the raw materials for synthesis is adopted here. Instead of the expensive pure-metal powders and scarce chemical reagents typical of self-propagating high-temperature synthesis, the new method employs ferroalloys, reducing agents, and other less expensive metallurgical products. For the first time, as a result, mass production based on self-propagating high-temperature synthesis is very economically efficient. Note that, in comparison with conventional furnace technologies, self-propagating high-temperature synthesis yields higher-quality products, with a new combination of operating properties. The introduction of ferrosilicon nitride produced by self-propagating high-temperature synthesis in Russia began with its successful use as an alloying additive in smelting grain-oriented steel with nitride inhibition. In the converter shop at OAO MMK, its use instead of nitrogen-bearing ferrochrome permitted practically 100 % increase in the nitrogen content of the steel. At NTPF Etalon Ltd, a special combined method for alloying with nitrogen was developed for the production of nitrogen-bearing transformer steel. In the new method, the melt is preliminarily saturated with nitrogen, in the form of ferrosilicon-nitride pieces, in the ladle, in the production of steel with around 0.006 % N. Finally, the nitrogen content is corrected by means of ferrosilicon nitride, which is preliminarily ground to powder, packed in a wire, and introduced during ladle treatment to obtain metal with 0.009-0.012 % N. Ferrosilicon nitride produced by self-propagating high-temperature synthesis (SHS ferrosilicon nitride), which is intended for the alloying of steel, contains less nitrogen: 18-23 %. Conversion to powder wire, whose filler contains less nitrogen, increases the assimilation of nitrogen and stabilizes the smelting of metal with narrow specified limits of nitrogen concentration. In addition, SHS ferrosilicon-nitride compositions of higher density and strength have been specially developed for use in chunk form in alloying in the ladle. The density of such chunks is 1.5-2.0 times that of the traditional furnace product. The strength of the chunks (10-50 MPa) is several times greater, and therefore dust formation may be almost completely eliminated when using the new material, with maximum increase in nitrogen assimilation by the steel. Note that a simple technology has been developed for chunk alloying in the ladle; this technology not only ensures high and stable nitrogen assimilation in the steel but also permits an additional 5-10 % reduction in the total silicon consumption, on account of more complete silicon assimilation from ordinary ferrosilicon. In contrast to the SHS ferrosilicon nitride used to alloy steel, the nitrogen concentration is higher in compositions used as additives in unshaped refractories. NTPF Etalon Ltd has developed ferrosilicon nitride with an increased nitrogen content and reduced iron concentration. A higher silicon-nitride concentration is ensured both by appropriate selection of the raw material and by choosing conditions that ensure a product with the maximum nitrogen content. Investigation of tap-hole and channel refractories containing SHS ferrosilicon nitride shows that the silicon nitride content must be at least 75 %. After industrial tests in 2006, the MMK blast-furnace shop has now completely converted to water-free refractories containing SHS ferrosilicon nitride. The introduction of the new refractories is associated with more prolonged hot-metal discharge and a greater discharge of smelting products. In addition, working conditions for the blast-furnace staff are much improved. Note that the properties of tap-hole refractories with SHS ferrosilicon nitride are better not only than those of traditional nitride-free refractories but those of materials containing ordinary furnace ferrosilicon nitride. The tabel 1 presents the compositions of the ferrosilicon nitride produced and supplied by NTPF Etalon Ltd. The products FSN35 - FSN25 are intended for the refractory industry. ОАО Spetsremstroy (Magnitogorsk) uses such ferrosilicon nitride to produce water-free tap-hole and trough materials of type SiO2-Al2O3-SiC-C. Mainly FSN15 - FSN25 are used for alloying; they ensure high and stable assimilation of nitrogen by the steel. Table 1. Chemical composition of ferrosilicon nitride produced at NTPF Etalon Ltd
Conclusions Thus, SHS technology for the production of a new material, ferrosilicon nitride, has been developed and industrially introduced by NTPF Etalon Ltd, and the first Russian system for the large-scale (in amounts of a few tons) production of metallurgical materials based on refractory inorganic compounds has been created. Russia has thus produced a technology for making a new refractory material based on NITRO-FESIL grade ferrosilicon nitride. The technology is based on SHS principles and enables one to produce the product without consuming electricity. This gives a product whose working characteristics are better than those of furnace analogs. Published in proceedings of "The Twelfth International Ferroalloys Congress", June 6-9, 2010, Helsinki, Finland (Article in pdf)
Produced by NTPF Etalon Ltd: |
||||||||||||||||||||||||||||||||||||||||||
