(3519) 580-155, mail@ntpf-etalon.ru
|
|
Ferrochromium Nitride SH-Synthesis. Part 2The previous article part - Ferrochromium Nitride SH-Synthesis. Part 1 PKh1S chromium powder produced by the calciumhydride method (Technical Specifications TU 14-1-1474–75) and PKhA97.5 chromium powder produced by the alumothermal method (Technical Specifications TU 14-00186482-051–2005) are used in laboratory experiments, as well as PFN low-carbon alumothermal ferrochrome (State Standard GOST 4757–91). The experimental method was described in; it is analogous to the nitriding of other metals and ferroalloys by combustion. The most important parameter of such combustion is the nitrogen pressure in the vicinity of the hot powder. In Fig. 1, the combustion rate of ferrochrome powder with 75.6% Cr is plotted as a function of the nitrogen pressure; for comparison, the analogous curves for standard alloys are shown: ferrotitanium (FTi70, 70.6 % Ti); ferrovanadium (FVa75U0.1; 71.6 % V); and ferroniobium (FN660; 60.6 % Nb). In all cases, powder with a particle size of <0.04 mm is used. The powder is burned without forced compaction in cylindrical gas-permeable casings of various diameters. The porosity of the initial samples is ~60 %. In all cases, the combustion rate increases with increase in the pressure, on account of the better conditions for nitrogen filtration. As would be expected, combustion is slowest for ferrochromium. This is due both to the less exothermal nitride formation for chromium than for metals of groups IV and V and to the fact that the reaction of ferrochrome and nitrogen in the combustion wave is not a solid-phase process. The melting point of low-carbon ferrochrome is 1550–1670 °C. Even if we take into account that the nitrogen dissolved in the ferrochrome somewhat reduces its melting point, this temperature will remain above the combustion temperature. In the given pressure range (1.0–12.0 MPa), the thermocouple readings of the combustion temperature are no higher than 1300 °C. Metallographic analysis of the combustion products confirms the absence of melting in the Cr–Fe–N system. 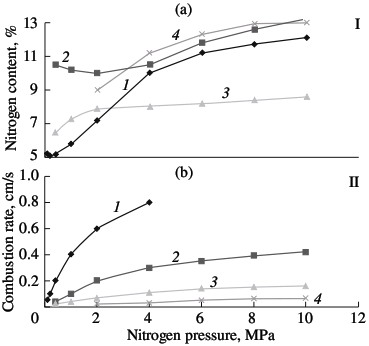 Fig. 1. Influence of the nitrogen pressure on the degree of nitriding (I) and combustion rate (II) of ferroalloys:
(1) ferrotitanium; (2) ferrovanadium; (3) ferroniobium; (4) ferrochrome. Solid-phase processes usually facilitate extensive nitriding of both metals and ferroalloys. The possibility of stage-by-stage saturation of the powder with nitrogen was observed earlier in the combustion of ferrovanadium and ferrosilicon. Most of the nitrogen is absorbed by the alloy in the synthesis wave, sometimes in conditions of laminar combustion. The unreacted portion of the ferroalloy reacts with nitrogen in conditions of bulk combustion. This facilitates the retention of high porosity at the combustion front. As a result, the nitrogen content in the products may be increase by 10–40 %, depending on the conditions of combustion and the composition of the initial alloy. Note, however, that, even in the most optimal combustion conditions, the maximum degree of nitriding is not attained in the nitriding of ferrochrome. For alloys with 75.6 % Cr, the limiting calculated nitrogen concentration when all the chromium is converted to nitride CrN1.0 is around 16.8 % N. The actual maximum nitrogen content in the ferrochrome is ~13.0 % N. Thus, the actual degree of nitriding is hardly more than 77 % of the calculated value. Close to 100 % of the maximum nitriding is obtained in the combustion of metallic chromium powder in nitrogen. The combustion rate and temperature and the degree of nitriding of calcium-hydride chromium powder with a particle size of <0.02 mm are plotted in Fig. 2. In the pressure range 0.1–0.9 MPa, all three parameters increase monotonically. At the maximum pressure, practically all the chromium has been converted to CrN. X-ray diffraction data show that the product obtained at pressures above 3.0 MPa consist of a single phase. At the minimum pressure (0.1–0.2 MPa), the burning material consists mainly of chromium seminitride Cr2N; lines corresponding to CrN and free chromium are also seen. With increase in pressure, the content of CrN rapidly rises. 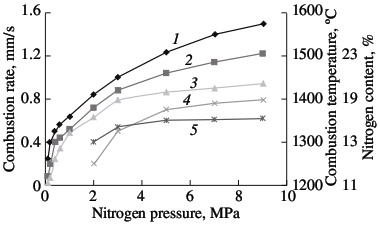 Fig. 2. Influence of the nitrogen pressure on the combustion rate (1, 4), degree of nitriding (3, 5) and combustion temperature (2):
(1, 2, 3) chromium; (4, 5) ferrochrome Alumothermal chromium powder is less active than calcium-hydride powder. The high reactivity is mainly a result of the large surface area of the powder, due to its small size. Microscopic analysis shows that, in calcium-hydride chromium, even relatively large particles (0.04–0.05 mm) in fact consist of aggregations of smaller particles (no greater than ~0.01 mm). At the same time, particles larger than 0.01 mm predominate in alumothermal chromium powder that passes through a 0.04-mm screen. An analogous pattern is observed for ferrochromium powder. On account of the high strength of the low-carbon ferrochrome and metallic chromium produced by the alumothermal method, considerable energy consumption is required in order to obtain fine powder. It is evident from Fig. 2 that the combustion rate and the degree of nitriding of alumothermal chromium powder are considerably less than those of calcium-hydride powder. Since the conversion depth of the alumothermal chromium is between 70.7 % (15.0 % N) and 82.1 % (17.4 % N), the combustion product consists of two phases, including two chromium nitrides. In contrast to chromium, the combustion products of ferrochrome always consist of multiple phases. X-ray diffraction data indicate α iron, a double nitride (Fe, Cr)2N, and the mononitride CrN. Over the whole pressure range, this phase composition remains basically the same. With increase in nitriding, thanks to higher pressure, the content of the mononitride and free iron increases. The same pattern was observed earlier in furnace synthesis. On the basis of the results of laboratory experiments and tests at NTPF Etalon Co., Ltd production facility (Magnitogorsk), an industrial system for the nitriding of chromium-based alloying additives by self-propagating high-temperature synthesis has been developed and introduced. Four fundamentally new high-nitrogen alloying materials are proposed here: sintered chromium nitride, fused chromium nitride, sintered ferrochromium nitride, and fused ferrochromium nitride. Table 3 presents the chemical and phase composition of these materials, as well as their density. The macrostructure of the new materials is shown in Fig. 3. Table 3. Chemical composition and properties of alloys produced by self-propagating high-temperature synthesis* 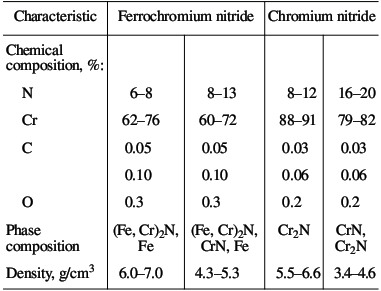 *The minimum content is given for nitrogen and chromium, while the maximum values are given for the other elements
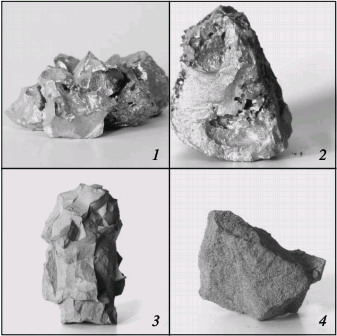 Fig. 3. Macrostructure of new alloying materials:
(1, 2) fused and sintered chromium nitride; (3, 4) fused and sintered ferrochromium nitride Ferrochromium nitride is used in smelting nitrogen-bearing corrosion-resistant steel and other chromium-bearing alloys with nitrogen. Chromium nitride is recommended for predominant use in smelting high-nitrogen chromonickel superalloys and also for high-alloy corrosion-resistant steel with the maximum nitrogen concentration. Fused ferrochromium nitride and chromium nitride may be effectively used in chunk form for direct alloying in the furnace or in the ladle at melt introduction. The sintered materials may be introduced not only in the traditional form but also as powder wire. On account of the high nitrogen concentration, the consumption of such wire will be minimal with high and stable nitrogen assimilation by the melt. The new nitrogen-bearing chromium materials have been successfully tested at numerous Russian and non-Russian plants in steel production. The required nitrogen content in the steel varies widely: from 0.04 % to 0.6 %. In particular, 12X18AГ18, 35X2AФ, 55X21Г9AH4, 110Г13XФAЛ, and related steel has been produced, as well as chromonickel alloy with ~0.5 % N. The metal is smelted in electrofurnaces, using various chromium and ferrochromium nitrides, containing 9.0–19.5 % N. In all cases, the degree of nitrogen assimilation is more than 90 %. Thus, industrial technology for the production of nitrogen-bearing chromium-based alloying materials on the basis of self-propagating high-temperature synthesis has been developed for the first time in Russia. The chromium-based alloying materials obtained at the Etalon production facility on the basis of self-propagating high-temperature synthesis have no counterparts anywhere in the world. Chromium and ferrochromium nitrides, which combine a high nitrogen concentration and maximum density, permit the economical smelting of a wide range of nitrogen steel. Published in "Steel In Translation" Volume 39, Number 9, September 2009 (article in pdf)
Ferrochromium and chromium nitride, produced by NTPF Etalon Co., Ltd: Other publications about SHS |
